The Food and Drug Administration (FDA) on Tuesday proposed a new rule that would require a front-of-package (FOP) nutrition label for most packaged foods and beverages. If finalized, the agency said the proposal would give consumers readily visible information about a food’s saturated fat, sodium and added sugars content, in addition to the standard nutrition labels on the back.
The FDA’s proposed front-of-package label would include the amount of saturated fat, sodium and added sugars and whether those amounts are considered “low,” “medium” or “high.”
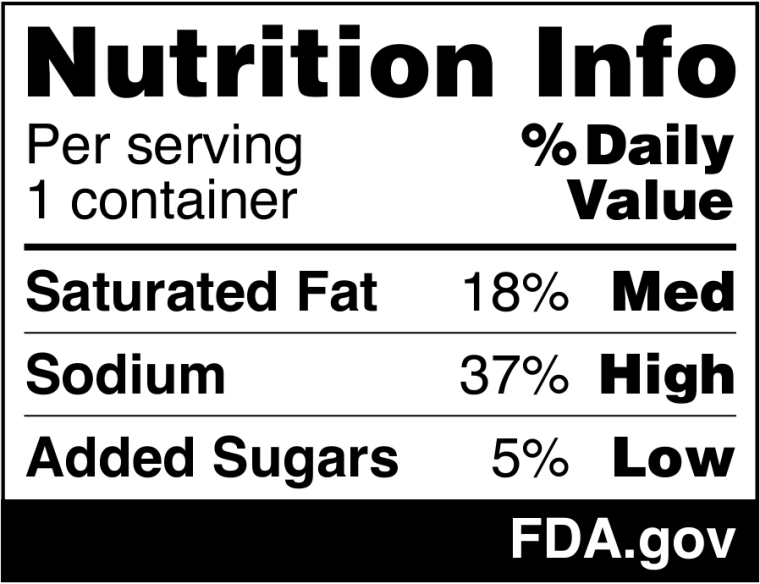
The proposed front-of-package labels would include saturated fat, sodium and added sugar, along with whether products contain high, medium or low amounts of the nutrients. U.S. Food & Drug Administration
The proposed rule, if finalized, would require food manufacturers to add a Nutrition Info box to most packaged food products three years after the final rule’s effective date for businesses with $10 million or more in annual food sales and four years after the final rule’s effective date for businesses with less than $10 million in annual food sales. The proposal includes a 120-day comment period, after which the agency may make additional changes to the proposal or finalize the new rule.
The Consumer Brands Association has opposed the mandatory labeling, saying the FDA is considering “schemes with arbitrary scales and symbols that could cause confusion among consumers.”
FMI – The Food Industry Association noted its concern that any action regarding labels be “done in a way that minimizes unnecessary costs while providing consumers with clear, consistent and science-based nutrition information. We also believe that reducing a food’s entire dietary contribution to whether it is low, medium or high in saturated fat, sodium and added sugar is overly simplistic and will not help educate consumers on how to improve their overall dietary pattern.”

